Buy Phenatine powder Cas 139-68-4
Buy Phenatine powder Cas 139-68-4
Phenatine, or phenatin, also known as N-nicotinoylamphetamine and sold under the brand name Fenatine, is a psychostimulant of the amphetamine family which was developed and used in the Soviet Union. It was used in the treatment of depression, narcolepsy, post-encephalitis sequelae, alcoholic psychoses, asthenia, and other conditions
Names and synonyms
Verified
139-68-4
[RN]
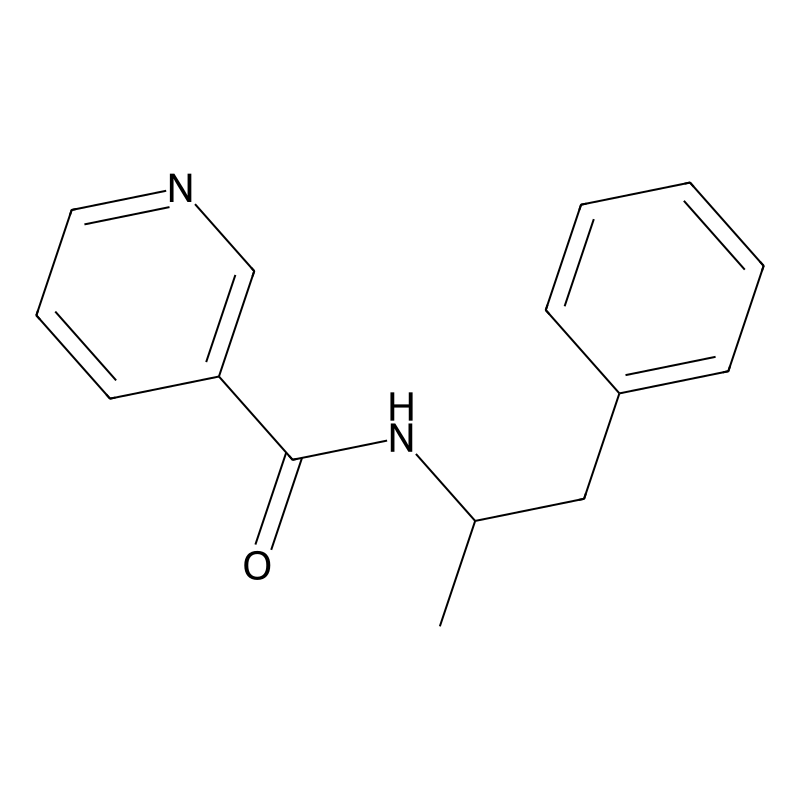
3-Pyridinecarboxamide, N-(1-methyl-2-phenylethyl)-
[ACD/Index Name]
N-(1-Phenyl-2-propanyl)nicotinamid
[German]
[ACD/IUPAC Name]
N-(1-Phenyl-2-propanyl)nicotinamide
[ACD/IUPAC Name]
N-(1-Phényl-2-propanyl)nicotinamide
[French]
[ACD/IUPAC Name]
N-(1-phenylpropan-2-yl)pyridine-3-carboxamide
N-(a-Methylphenethyl)nicotinamide
Nicotinic Acid b-Phenylisopropylamide
Nicotinoyl-b-phenylisopropylamine
phenatine
Unverified Buy Phenatine powder Cas 139-68-4
3-Pyridinecarboxamide, N- (1-methyl-2-phenylethyl)-
3-Pyridinecarboxamide, N-(1-methyl-2-phenylethyl)- (9CI)
Fenatin
N(α-Methylphenethyl)nicotinamide
N-(1-Methyl-2-phenylethyl)-3-pyridinecarboxamide
N-(1-Methyl-2-phenylethyl)nicotinamide
N-(α-Methylphenethyl)nicotinamide
Nicotinamide, N- (α-methylphenethyl)-
Nicotinamide, N-(α-methylphenethyl)-
Nicotinic acid β-phenylisopropylamide
Nicotinoyl-β-phenylisopropylamine
Perviton
Phenatin
An analogue is methylphenatine. Another analogue is pyridoxiphen (pyridoxiphene; pyridoxylamphetamine), the condensation product of amphetamine and pyridoxine (vitamin B6).[5][15][16] Other relatives may include thiophenatine, propylphenamine, and phenylphenamine.[13] Further analogues of phenatine and pyridoxphen include gamofen (the condensation product of amphetamine and γ-aminobutyric acid (GABA)), alafen (the condensation product of amphetamine and β-alanine), and pabofen (p-aminobenzoylamphetamine; linkage of amphetamine and p-aminobenzoic acid (PABA), an intermediate in the biosynthesis of folic acid (vitamin B9)), all of which show distinct pharmacological activity
Description
Phenatine is a central nervous system (CNS) stimulant with the chemical formula C₁₅H₁₆N₂O, described as a colorless crystalline or white powder that is soluble in water and ethanol but nearly insoluble in ether . Notably, lists “this compound” as a historical synonym for phenytoin, an antiepileptic drug; however, this appears to be a nomenclature conflict, as clearly distinguishes this compound as a distinct compound with stimulant properties. This discrepancy underscores the importance of verifying chemical identities in cross-referencing literature .
Properties
CAS No. |
139-68-4 |
|---|---|
Molecular Formula |
C15H16N2O |
Molecular Weight |
240.3 g/mol |
IUPAC Name |
N-(1-phenylpropan-2-yl)pyridine-3-carboxamide |
InChI |
InChI=1S/C15H16N2O/c1-12(10-13-6-3-2-4-7-13)17-15(18)14-8-5-9-16-11-14/h2-9,11-12H,10H2,1H3,(H,17,18) |
InChI Key |
KJRJJAZBUWXZFN-UHFFFAOYSA-N |
SMILES |
CC(CC1=CC=CC=C1)NC(=O)C2=CN=CC=C2 |
Canonical SMILES |
CC(CC1=CC=CC=C1)NC(=O)C2=CN=CC=C2 |
Other CAS No. |
139-68-4 |
Synonyms |
Fenatin Fencarol Phencarol quifenadine quifenadine hydrobromide, (+-)-isomer quifenadine hydrochloride quinuclidinyl-3-diphenylcarbinol |
Origin of Product |
United States |
Scientific Research Applications
Pharmacological Mechanism of Action
Phentermine acts primarily as an appetite suppressant through its influence on neurotransmitter release. It promotes the release of norepinephrine in the brain, which subsequently reduces hunger signals. The pharmacodynamics of phentermine indicate that it is more selective for norepinephrine than for dopamine or serotonin, making it a unique agent among weight loss medications .
Table 1: Mechanism of Action of Phentermine
| Mechanism | Description |
|---|---|
| Norepinephrine Release | Enhances norepinephrine levels, leading to appetite suppression. |
| Dopamine Interaction | Less potent in dopamine release compared to norepinephrine. |
| Serotonin Interaction | Minimal effect on serotonin levels at therapeutic doses. |
Clinical Applications in Obesity Management
Phentermine is predominantly used in conjunction with diet and exercise to facilitate weight loss in obese patients. Clinical trials have demonstrated significant weight reduction associated with phentermine treatment.
Clinical Trial Findings
A review of multiple clinical trials reveals that phentermine can lead to an average weight loss of approximately 3.6 kg compared to placebo over a period of 12 weeks . Notably, a study involving 108 obese women showed that those receiving continuous phentermine therapy lost an average of 12.2 kg over 36 weeks .
Table 2: Summary of Clinical Trials on Phentermine
| Study Name | Duration | Participants | Weight Loss (kg) | Notes |
|---|---|---|---|---|
| Munro et al. | 36 weeks | 108 women | 12.2 (continuous) | Significant weight reduction compared to placebo. |
| Weintraub et al. | 24 weeks | 81 subjects | 10 (phentermine) | Compared effects with fenfluramine and placebo. |
| Langlois et al. | 22 weeks | 59 patients | 16.1 | Demonstrated superior weight loss vs placebo. |
Emerging Research and Future Directions
Recent studies have explored the potential for combining phentermine with other agents such as topiramate to enhance weight loss outcomes further. The combination therapy has shown promise in clinical settings, suggesting a synergistic effect that may improve efficacy while maintaining safety profiles .
Chemical Reactions Analysis
Step 1: Formation of N-(1,1-dimethyl-phenethyl)acetamide
- Reactants : Dimethylbenzyl carbinol, acetic acid, sulfuric acid (catalyst), and acetonitrile/propionitrile/n-butyronitrile as solvents.
- Conditions :
- Ice-water bath (0–4°C).
- Stirring for 2–15 hours.
- pH adjustment to 6–8 using KOH/NaOH/Ba(OH)₂.
- Yield : 94.92% (reported in optimized conditions) .
Step 2: Hydrolysis to Phentermine Free Base
- Reactants : N-(1,1-dimethyl-phenethyl)acetamide, inorganic strong base (KOH/NaOH), and solvents (ethylene glycol, DMSO).
- Conditions :
- Heating to reflux (5–15 hours).
- Extraction with ethyl acetate and drying with anhydrous sodium sulfate.
- Vacuum distillation to isolate phentermine free base.
- Yield : 97.78% .
Acid-Catalyzed Amidation
The initial step involves sulfuric acid acting as a catalyst to promote the reaction between dimethylbenzyl carbinol and acetic acid, forming an acetamide intermediate. This proceeds via nucleophilic acyl substitution .
Base-Mediated Hydrolysis
The hydrolysis of the acetamide intermediate to phentermine free base employs strong bases (e.g., NaOH) under reflux. This reaction likely follows an SN2 mechanism, with the base deprotonating the amide and facilitating cleavage of the acetyl group . Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4, Buy Phenatine powder Cas 139-68-4
Metabolic Reactions
Phentermine undergoes limited hepatic metabolism (~6% of the dose) :
- Primary Pathways :
- P-Hydroxylation : Formation of p-hydroxy-phentermine.
- N-Oxidation : Generation of N-hydroxyphentermine.
- Conjugation : Glucuronidation of metabolites.
| Metabolic Reaction | Enzyme Involved | Major Metabolite |
|---|---|---|
| P-Hydroxylation | CYP2D6 | p-Hydroxy-phentermine |
| N-Oxidation | Flavin-containing monooxygenases | N-Hydroxyphentermine |
Degradation and Stability
Phentermine hydrochloride is stable under standard storage conditions but degrades under strongly acidic or basic conditions :
- Hydrolysis : Rapid decomposition in aqueous solutions with pH < 2 or > 12.
- Thermal Stability : Stable up to 200°C, with decomposition products including styrene derivatives .
Comparative Reaction Kinetics
High-throughput experimentation (HTE) analyses of analogous amines reveal:
- Solvent Dependence : Reactions in polar aprotic solvents (e.g., DMSO) show higher yields due to improved stabilization of transition states .
- Catalyst Impact : Bulky ligands (e.g., BrettPhos) enhance reductive elimination in cross-coupling reactions, though phentermine synthesis avoids such complexity .
Q & A
How to formulate a research question exploring this compound’s structure-activity relationships (SAR)?
- Methodological Answer : Apply the PICO framework (Population: target receptor; Intervention: this compound analogs; Comparison: baseline activity; Outcome: binding affinity). For example: “How do electron-withdrawing substituents on this compound’s aryl ring affect its inhibition of [Target X] compared to unmodified this compound?” . Validate feasibility using molecular docking simulations before wet-lab experiments .
Advanced Research Questions
Q. How to resolve contradictions in this compound’s reported pharmacokinetic data across studies?
- Methodological Answer : Conduct a meta-analysis to identify confounding variables (e.g., dosing regimens, animal models). Use statistical tools (e.g., Cohen’s d for effect size heterogeneity) to assess bias . Replicate disputed experiments under standardized conditions, and validate findings via blind testing or third-party labs . Publish negative results to clarify discrepancies .
Q. What strategies mitigate batch-to-batch variability in this compound production for in vivo studies?
- Methodological Answer : Implement Quality-by-Design (QbD) principles:
- Define Critical Quality Attributes (CQAs: purity, particle size) and Critical Process Parameters (CPPs: mixing speed, drying time) .
- Use Design of Experiments (DoE) to model interactions between variables. For example, a 2^3 factorial design to optimize crystallization conditions .
- Share raw data and SOPs in supplementary materials to enable cross-lab verification .
Q. How to integrate multi-omics data (e.g., transcriptomics, metabolomics) to elucidate this compound’s mechanism of action?
- Methodological Answer :
- Data Integration: Use pathway enrichment tools (e.g., KEGG, Reactome) to identify overlapping pathways affected by this compound .
- Validation: Apply CRISPR-Cas9 knockouts of top candidate genes in cell models, followed by phenotypic rescue assays .
- Contradiction Management: Prioritize findings replicated in ≥2 independent datasets and exclude platform-specific artifacts (e.g., batch effects in microarray vs. RNA-seq) .
Q. What ethical and methodological considerations apply when designing clinical trials for this compound derivatives?
- Methodological Answer :
- Ethics: Align with CONSORT guidelines for randomized trials, including informed consent and data anonymization .
- Bias Mitigation: Use double-blinding, placebo controls, and stratified randomization by demographic factors (age, comorbidities) .
- Data Transparency: Pre-register trials on ClinicalTrials.gov and share adverse event logs .
Comparison with Similar Compounds
Structural and Pharmacological Profiles
The table below compares Phenatine with three compounds from the same evidence source (Clarke’s Analysis of Drugs and Poisons), highlighting differences in chemical structure, pharmacological class, and primary uses:
| Compound | Chemical Formula | CAS Number | Pharmacological Class | Primary Use | Key Properties |
|---|---|---|---|---|---|
| This compound | C₁₅H₁₆N₂O | Not provided | CNS Stimulant | Stimulant (specifics unknown) | Soluble in water, ethanol; crystalline form |
| Phenamidine | C₁₄H₁₄N₄O | 101-62-2 | Antiprotozoal | Treatment of babesiosis in animals | Administered as isethionate salt; hepatotoxic at high doses |
| Phenampromide | C₁₇H₂₆N₂O | Not provided | Analgesic | Pain relief (opioid-like) | (-)-isomer exhibits higher analgesic potency |
| Phenazocine | C₂₂H₂₇NO | 127-35-5 | Opioid Analgesic | Moderate to severe pain management | Hydrobromide salt form; structurally distinct from this compound |
Key Observations :
- Its stimulant properties contrast sharply with the sedative or analgesic effects of the others.
- Therapeutic Targets : this compound’s CNS stimulation diverges from Phenamidine’s antiparasitic action and the pain-focused applications of Phenampromide/Phenazocine.
Nomenclature and Regulatory Considerations
lists this compound under 苯甲酸酯类 (benzoate esters), alongside compounds like phenazocine hydrobromide and phentermine salts. However, this classification is inconsistent with , which categorizes this compound as a standalone stimulant. Regulatory documents () emphasize the need for precise chemical identification to avoid confusion in drug approval and labeling processes. For example, Phenytoin (synonymously called “this compound” in some contexts) is a sodium channel blocker used for epilepsy, highlighting the risks of ambiguous nomenclature .
Phenatine
Content Navigation
CAS Number
Product Name
IUPAC Name
Molecular Formula
Molecular Weight
InChI
InChI Key
SMILES
Synonyms
Canonical SMILES
Description
Phenatine, commonly known as phentermine, is a sympathomimetic amine that functions primarily as an appetite suppressant. It is chemically classified as a substituted amphetamine, specifically α,α-dimethylphenethylamine. First approved for medical use in 1959, phentermine is primarily prescribed for short-term weight management in conjunction with lifestyle modifications such as diet and exercise. It acts by stimulating the central nervous system, leading to increased release of catecholamines like norepinephrine, which suppress appetite and promote fat breakdown .
Phentermine undergoes several metabolic transformations within the body. The primary metabolic pathways include:
- p-Hydroxylation: A minor pathway resulting in the formation of p-hydroxyphentermine.
- N-Oxidation and N-Hydroxylation: These reactions yield N-oxidized and N-hydroxylated metabolites.
- Conjugation: Following oxidation, these metabolites may undergo conjugation.
Approximately 6% of the administered dose is metabolized, with the majority excreted unchanged in urine (70-80%) .
Phentermine can be synthesized through various methods involving the alkylation of phenethylamine derivatives. A common synthetic route includes:
- Starting Material: Phenethylamine.
- Alkylation: The introduction of two methyl groups at the alpha carbon using methyl iodide or dimethyl sulfate under basic conditions.
- Purification: The crude product is purified through recrystallization or chromatography.
This method yields phentermine hydrochloride as a white crystalline powder that is soluble in water and alcohols .
Phentermine interacts with several medications and substances:
- Monoamine Oxidase Inhibitors (MAOIs): Co-administration can lead to hypertensive crises.
- Antihypertensives: Phentermine may reduce their effectiveness.
- Stimulants: Increased risk of cardiovascular side effects when combined with other stimulants .
These interactions necessitate careful monitoring when prescribing phentermine alongside other medications.
Phenatine shares structural and functional similarities with other compounds in the amphetamine class. Here are some comparable compounds:
| Compound Name | Chemical Structure | Primary Use | Unique Features |
|---|---|---|---|
| Amphetamine | C9H13N | ADHD treatment | Stronger CNS stimulant effects |
| Methamphetamine | C10H15N | ADHD treatment; obesity | Higher potential for abuse |
| Dexamphetamine | C9H13N | ADHD treatment | Stereoisomer of amphetamine |
| Methylphenidate | C14H19NO2 | ADHD treatment | Different mechanism of action |
| Sibutramine | C17H26ClN | Obesity management | Serotonin reuptake inhibitor |
Uniqueness of Phenatine
Phenatine’s uniqueness lies in its specific mechanism of action as a norepinephrine releasing agent with relatively lower abuse potential compared to methamphetamine and its derivatives. While it shares pharmacological properties with other stimulants, its primary role as an appetite suppressant distinguishes it within the class of sympathomimetics .




Reviews
There are no reviews yet.