Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0
Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0
1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol, sometimes misleadingly referred to as hydroxylimine hydrochloride, is a chemical compound which is the final intermediate in the synthesis of ketamine, an anaesthetic drug which is also subject to recreational abuse. This chemical intermediate is not active as a drug in its own right, and is legal in most countries, but is readily converted into ketamine by dissolving it in a suitable high-boiling point solvent and heating, with no other chemicals required.[1][2] This has made it subject to illicit trade as a drug precursor, and it has sometimes been seized by law enforcement agencies in significant quantities, leading to it being specifically banned as a controlled drug precursor in some jurisdictions such as Taiwan Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0
Synthesis Analysis
The synthesis of 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol typically involves the following steps:
- Reaction of 2-Chlorobenzonitrile with Cyclopentanone:
- A common method involves reacting 2-chlorobenzonitrile with cyclopentanone in the presence of a base (such as sodium ethoxide) to form an imine intermediate.
- This reaction requires careful control of temperature and reaction time to optimize yield.
- Formation of the Cyclopentanol Derivative:
- The imine can then be reduced to form the desired cyclopentanol derivative, often using reducing agents like lithium aluminum hydride . Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0
- Final Steps:
- The final product can be purified through recrystallization or chromatography techniques to ensure high purity suitable for further applications.
Molecular Structure Analysis
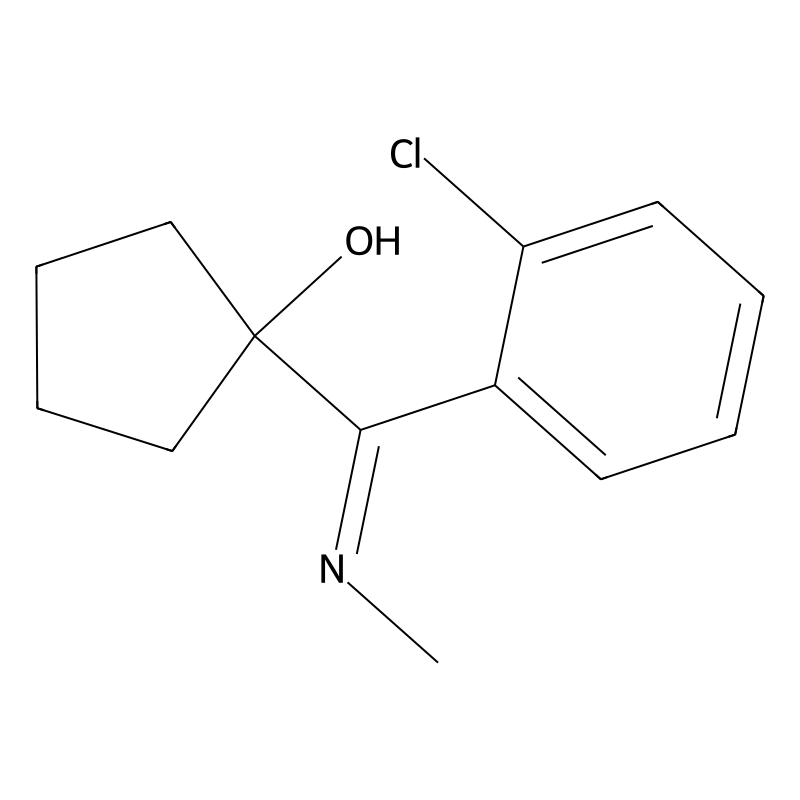
The molecular structure of 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol features several key functional groups:
- Cyclopentanol Ring: A five-membered carbon ring with a hydroxyl group (-OH), indicating alcohol functionality.
- Chlorinated Benzene Ring: A benzene ring substituted with a chlorine atom at the para position relative to the nitrogen atom.
- Amide Linkage: The nitrogen atom connects the chlorinated benzene and cyclopentanol components through a carbonyl group, forming an amide structure.
The specific arrangement of these groups influences the reactivity and stability of the molecule, making it suitable for ketamine synthesis .
Chemical Reactions Analysis Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0
1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol participates in various chemical reactions:
- Oxidation: This compound can be oxidized to yield corresponding ketones or carboxylic acids under specific conditions, typically using oxidizing agents like potassium permanganate .
- Reduction: Reduction reactions can convert it into different amines or alcohols, depending on the reagents used.
- Substitution Reactions: It can undergo nucleophilic substitution where the chlorine atom is replaced by other nucleophiles such as amines or alcohols.
These reactions are vital for modifying the compound for various synthetic pathways in organic chemistry.
Mechanism of Action
The mechanism of action for 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol primarily revolves around its conversion to ketamine:
- Conversion Process: When dissolved in a high-boiling point solvent (e.g., ethanol) and heated, 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol undergoes a transformation into ketamine without requiring additional reagents .
- Environmental Factors: The efficiency of this conversion can be influenced by factors such as temperature and solvent choice, which affect the reaction kinetics and yield.
Physical and Chemical Properties Analysis
The physical and chemical properties of 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol include:
These properties are essential for handling and application in laboratory settings.
Applications
1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol has several scientific applications:
- Anesthetic Pharmacology: It serves as an essential intermediate in synthesizing ketamine, which is valuable in anesthesia due to its rapid onset and minimal respiratory depression .
- Analytical Chemistry: Used as a reference material for developing analytical methods to detect ketamine and its impurities through techniques like high-performance liquid chromatography (HPLC) .
- Forensic Toxicology: Relevant in forensic investigations where detection of ketamine use or abuse is necessary. Its presence in biological samples can indicate exposure or overdose cases .
Properties
CAS Number
Product Name
IUPAC Name
Molecular Formula
Molecular Weight
InChI
InChI Key
SMILES
Synonyms
Canonical SMILES
There total 14 articles about 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol which guide to synthetic route it. The literature collected by LookChem mainly comes from the sharing of users and the free literature resources found by Internet computing technology. We keep the original model of the professional version of literature to make it easier and faster for users to retrieve and use. At the same time, we analyze and calculate the most feasible synthesis route with the highest yield for your reference as below:
1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol
- Chemical Name:1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol
- CAS No.:6740-87-0
- Molecular Formula:C13H16ClNO
- Molecular Weight:237.729
- Hs Code.:2925294500
- European Community (EC) Number:641-894-8,936-422-5
- UNII:HGI99H315N
- DSSTox Substance ID:DTXSID60217758
- Nikkaji Number:J22.353K
- Wikidata:Q27279915
- Wikipedia:1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol
- Mol file:6740-87-0.mol
Synonyms:6740-87-0;1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol;UNII-HGI99H315N;HGI99H315N;Cyclopentanol, 1-((2-chlorophenyl)(methylimino)methyl)-;1-[C-(2-chlorophenyl)-N-methylcarbonimidoyl]cyclopentan-1-ol;Ketamine hydrochloride specified impurity A [EP];1-[(2-chlorophenyl)(methylimino)methyl]cyclopentanol;1-((2-CHLOROPHENYL)-(METHYLIMINO)METHYL)CYCLOPENTANOL;1-[(2-Chlorophenyl)-(methylimino)methyl]cyclopentanol;Ketamine hydroxylimine precursor;SCHEMBL21081869;SCHEMBL21081870;SCHEMBL21133129;DTXSID60217758;PD133719;PD148989;KETAMINE HYDROCHLORIDE IMPURITY A [EP IMPURITY];Q27279915;ESKETAMINE HYDROCHLORIDE IMPURITY A [EP IMPURITY];1-[(2-CHLOROPHENYL)(METHYLIMINO)METHYL]CYCLOPENTAN-1-OL
 This product is a nationally controlled contraband, and the Lookchem platform doesn’t provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn’t provide relevant sales information.
- Vapor Pressure:0.03-0.061Pa at 20-25℃
- Melting Point:64-65 °C
- Boiling Point:347.3±42.0 °C(Predicted)
- PKA:13?+-.0.20(Predicted)
- PSA:32.59000
- Density:1.18±0.1 g/cm3(Predicted)
- LogP:3.06400
- Storage Temp.:2-8°C
- XLogP3:2.8
- Hydrogen Bond Donor Count:1
- Hydrogen Bond Acceptor Count:2
- Rotatable Bond Count:2
- Exact Mass:237.0920418
- Heavy Atom Count:16
- Complexity:271
- Purity/Quality:
- Pictogram(s):
1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol
Content Navigation
CAS Number
Product Name
IUPAC Name
Molecular Formula
Molecular Weight
InChI
InChI Key
SMILES
Synonyms
Canonical SMILES
Anesthetic Pharmacology
Specific Scientific Field: Anesthetic pharmacology focuses on understanding and developing drugs that induce anesthesia, leading to loss of sensation and consciousness during medical procedures.
Application Summary: 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol serves as an essential intermediate in the synthesis of ketamine, a widely used anesthetic. Ketamine is known for its rapid onset and unique properties, making it valuable in both human and veterinary medicine.
Experimental Procedures:Synthesis: Researchers synthesize 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol using appropriate chemical reactions.
Conversion to Ketamine: The compound is then converted into ketamine by dissolving it in a suitable high-boiling point solvent (e.g., ethanol) and heating. No additional chemicals are required for this conversion.
Results and Outcomes: The successful synthesis of ketamine from 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol leads to a potent anesthetic agent. Ketamine is used in surgical procedures, pain management, and as an alternative to opioids. Its dissociative effects and minimal respiratory depression make it particularly valuable in emergency medicine and pediatric anesthesia .
Analytical Chemistry
Specific Scientific Field: Analytical chemistry involves the study of methods and techniques to identify, quantify, and characterize chemical compounds.
Application Summary: Researchers use 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol as a reference material in analytical laboratories. It aids in the development and validation of analytical methods for detecting and quantifying ketamine and related compounds.
Experimental Procedures:Calibration Standards: Scientists prepare calibration standards containing known concentrations of 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol.
Instrumental Analysis: High-performance liquid chromatography (HPLC), gas chromatography (GC), or mass spectrometry (MS) techniques are employed to analyze samples containing ketamine and its impurities.
Results and Outcomes: Accurate quantification of ketamine in pharmaceutical formulations, biological samples, or seized materials ensures drug quality control, forensic investigations, and compliance with regulatory standards .
Forensic Toxicology
Specific Scientific Field: Forensic toxicology examines the presence of drugs and toxins in biological samples for legal and investigative purposes.
Application Summary: 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol is relevant in forensic toxicology due to its role as a precursor to ketamine. Detecting this compound in biological matrices helps identify ketamine abuse or exposure.
Experimental Procedures:Sample Extraction: Biological samples (e.g., urine, blood) are extracted using appropriate methods.
Analysis: Liquid chromatography-tandem mass spectrometry (LC-MS/MS) detects 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol and its metabolites.
Results and Outcomes: Identification of this compound assists in assessing ketamine use, overdose cases, and drug-related fatalities .
1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol is a chemical compound with the molecular formula C₁₃H₁₆ClNO and a molar mass of 237.73 g·mol⁻¹. It is an important intermediate in the synthesis of ketamine, a widely used anesthetic that has gained attention for its recreational misuse. The compound is not pharmacologically active by itself but can be readily converted into ketamine through simple chemical processes involving heating in a suitable solvent . Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0, Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Cas 6740-87-0
The primary reaction involving 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol is its conversion into ketamine. This process typically requires dissolving the compound in a high-boiling point solvent and heating it, which facilitates the transformation without the need for additional reagents .
Reaction Overview
- Starting Compound: 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol
- Reagents: High-boiling point solvent (e.g., dimethyl sulfoxide)
- Conditions: Heating
- Product: Ketamine
While 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol itself does not exhibit significant biological activity, its derivative, ketamine, has various pharmacological effects. Ketamine acts primarily as an NMDA receptor antagonist and is utilized in both human and veterinary medicine for its anesthetic properties. Additionally, ketamine has been studied for its rapid antidepressant effects and potential use in treating mood disorders .
The synthesis of 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol typically involves multi-step organic reactions, often starting from simpler precursors.
Common Synthesis Steps
- Formation of Benzimidazole Derivative: The initial step may involve the synthesis of a benzimidazole derivative that contains the chloro and methyl substituents.
- Cyclization: The benzimidazole derivative is then cyclized with cyclopentanol to form the final product.
- Purification: The crude product is purified through recrystallization or chromatography.
This compound can also be synthesized using variations of existing methods for producing ketamine, reflecting its role as an intermediate .
Several compounds share structural or functional similarities with 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol, particularly those involved in the synthesis of ketamine or related substances.
| Compound Name | Structure Similarity | Uses |
|---|---|---|
| Ketamine | Direct derivative | Anesthetic, antidepressant |
| Norketamine | Structural analog | Active metabolite of ketamine |
| 2-(2-Chlorophenyl)-2-nitrocyclohexanone | Precursor to norketamine | Potential illicit synthesis |
Uniqueness
1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol is unique due to its specific role as an intermediate in ketamine synthesis and its legal status as a non-controlled substance despite being linked to illicit drug production. Its structure allows for straightforward conversion into a clinically significant compound without requiring complex reagents or conditions .
UNII
GHS Hazard Statements
H302 (50%): Harmful if swallowed [Warning Acute toxicity, oral];
H318 (50%): Causes serious eye damage [Danger Serious eye damage/eye irritation];
H411 (50%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard];
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Pictograms



Corrosive;Irritant;Environmental Hazard
Other CAS
Dates
-
1- (2-Chloro-N-methylbenzimidoyl)cyclopentanol
1- (2-Chloro-N-methylbenzimidoyl)cyclopentanol | C13H16ClNO | CID 13086940 – structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, …
-
Buy 1- (2-Chloro-N-methylbenzimidoyl)cyclopentanol (EVT …
1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol has several scientific applications: Anesthetic Pharmacology: It serves as an essential intermediate in synthesizing ketamine, which is …
-
1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol – LookChem
Chemical Name: 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol; CAS No.: 6740-87-0; Molecular Formula: C13H16ClNO; Molecular Weight: 237.729; Hs Code.: 2925294500; European …
-
Ketamine Related Compound A – MilliporeSigma
1- [ (2-Chlorophenyl) (methylimino)methyl]cyclopentanol, 1- (2-Chloro-N-methylbenzimidoyl)cyclopentanol, Ketamine hydrochloride Impurity A (PhEur) Looking for …
-
Brand: SIAL
-
-
1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol 6740-87-0 – ECHEMI
Contact China Trader Xiamen Eagle Chemical Corporation for the product 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol 6740-87-0. Chat now for more business.
-
1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol Shanghai Macklin …
Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol,CAS 6740-87-0. Samples are available for international shipping.
-
Mendel Chemicals SRL
Synonym: 1-[(2-Chlorophenyl)(methylimino)methyl]cyclopentanol, 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol, Ketamine hydrochloride Impurity A (PhEur) Molecular …
-
Buy 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol | 6740-87-0
1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol is a chemical compound with the molecular formula C₁₃H₁₆ClNO and a molar mass of 237.73 g·mol⁻¹. It is an important intermediate in the …
-
1-2-CHLORO-N-METHYLBENZIMIDOYL-CYCLOPENTANOL
List of pharmaceutical 1-2-CHLORO-N-METHYLBENZIMIDOYL-CYCLOPENTANOL APIs marketplace enquires related to manufacturers, suppliers & exporters enquires available on …




Reviews
There are no reviews yet.